AtaGenix Laboratories
AtaGenix Laboratories

2025-10-13
AtaGenix Custom Proteins Services Enable Breakthrough in Understanding Cholangiocarcinoma Drug Resistance
This study identifies that ROCK2 promotes Pemigatinib resistance in CCA cells via UBA52-mediated DRP1 ubiquitination. AtaGenix provided high-purity custom proteins essential for validating this mechanism.
2025-07-21
AtaGenix Custom rMet Protein Advances Diabetes β-Cell Research
AtaGenix developed high-purity, endotoxin-free recombinant METRNL protein for research teams, uncovering its critical role in preventing β-cell trans-differentiation to α-cells and sustaining islet function, advancing diabetes metabolic stress studies, and offering new therapeutic target insights.
2025-06-20
AtaGenix Provides Custom gB and gB H516P Protein Expression Services for Chinese Academy of Medical Sciences’ HSV-1 Vaccine Research
AtaGenix Laboratories Co., Ltd. partnered with the Chinese Academy of Medical Sciences (Kunming) to provide custom protein expression services for an HSV-1 vaccine study published in Vaccine (DOI: 10.1016/j.vaccine.2025.127241). The study evaluated the immunogenicity of HSV-1 glycoprotein B (gB) and its H516P prefusion mutant in mRNA and subunit vaccines, finding no significant difference in immune responses. AtaGenix expressed gB and gB H516P proteins using Chinese hamster ovary (CHO) cells in a mammalian cell expression system and purified them via nickel column chromatography. Validated through ELISpot and ELISA, these high-purity proteins supported the study’s findings on the efficacy of QS-21 and CpG ODNs adjuvants in eliciting cellular immune responses, advancing HSV-1 vaccine development.
2025-06-20
AtaGenix Delivers Custom RMP and IKKβ Protein Expression Services for Naval Medical University’s Sepsis Research
AtaGenix Laboratories Co., Ltd. partnered with Naval Medical University researchers to provide custom protein expression services for a sepsis study published in Cell Communication and Signaling (DOI: 10.1186/s12964-025-02278-w). The study demonstrated that RNA polymerase II subunit 5-mediating protein (RMP) inhibits TLR4-induced NF-κB signaling in macrophages by binding IκB kinase β (IKKβ) and recruiting protein phosphatase 2A (PP2A). AtaGenix delivered high-purity GST, GST-mouse RMP-WT, GST-mouse RMP-S439A, and His-mouse IKKβ proteins using an E. coli expression system, validated via Western blot (WB). These proteins supported surface plasmon resonance (SPR), GST-pulldown, and ADP-Glo kinase assays, confirming RMP’s role in modulating inflammation and its potential as a therapeutic target for sepsis.
2025-06-20
AtaGenix Provides Custom PRPF19 Protein Expression Services for Huazhong University of Science and Technology’s Diabetic Nephropathy Research
AtaGenix Laboratories Co., Ltd. partnered with researchers at Huazhong University of Science and Technology to provide custom PRPF19 protein expression services for a study on diabetic nephropathy (DN) published in Cell Communication and Signaling (DOI: 10.1186/s12964-025-02253-5). The study elucidated the role of PRPF19 in mediating ubiquitin-dependent degradation of vitamin D receptor (VDR), exacerbating ferroptosis in renal tubular epithelial cells (RTECs). AtaGenix delivered high-purity PRPF19 protein (1.67 mg/ml) for surface plasmon resonance (SPR) studies, validated through Western blot (WB) and immunoprecipitation (IP) assays. The protein enabled the team to confirm berberine (BBR) as a PRPF19 inhibitor, offering a novel therapeutic approach to mitigate DN by reducing ferroptosis.
2025-05-30
Professional Protein Expression and Antibody Customization Services to Support High-Impact Research on UGT Gene Family in Platycodon grandiflorus
A study identified 107 UGT genes in Platycodon grandiflorus, with PgUGT29 catalyzing the glycosylation of Platycodin D to D3. AtaGenix provides tailored protein expression and antibody customization, ensuring high-purity PgUGT29 (>95%) and specific anti-PgUGT29 antibodies for functional validation via HPLC and molecular docking, advancing saponin biosynthesis research.
2025-05-30
Professional Protein Expression and Antibody Customization Services for Grass Carp C5a-CP Adjuvant Research
Grass carp C5a-CP shows exceptional potential as a vaccine adjuvant, enhancing IgM⁺ B cell phagocytic activity and vaccine efficacy. We provide professional protein expression and antibody customization services, including IgM-CH expression and purification and anti-C5aR polyclonal antibody production, supporting functional validation with high purity and specificity to advance fish vaccine adjuvant research.
2024-11-05
Stable Cell Line Development for Antibodies and Viral Proteins | AtaGenix Case Studies
Explore AtaGenix case studies on recombinant protein expression, including CHO stable cell lines achieving >3 g/L antibody yields and Bacillus subtilis systems for Protein A and B production. Data validated by SDS-PAGE, stability, purity, and affinity assays highlight AtaGenix’s expertise in high-quality protein expression solutions.
2024-11-05
Protein A and Protein B Expression in Bacillus subtilis | High-Purity Recombinant Protein Solutions by AtaGenix
Discover how AtaGenix optimized Protein A secretion and Protein B intracellular expression in Bacillus subtilis. Case studies show high yields and >90% purity validated by SDS-PAGE, highlighting AtaGenix’s expertise in recombinant protein expression and quality control.
2024-11-05
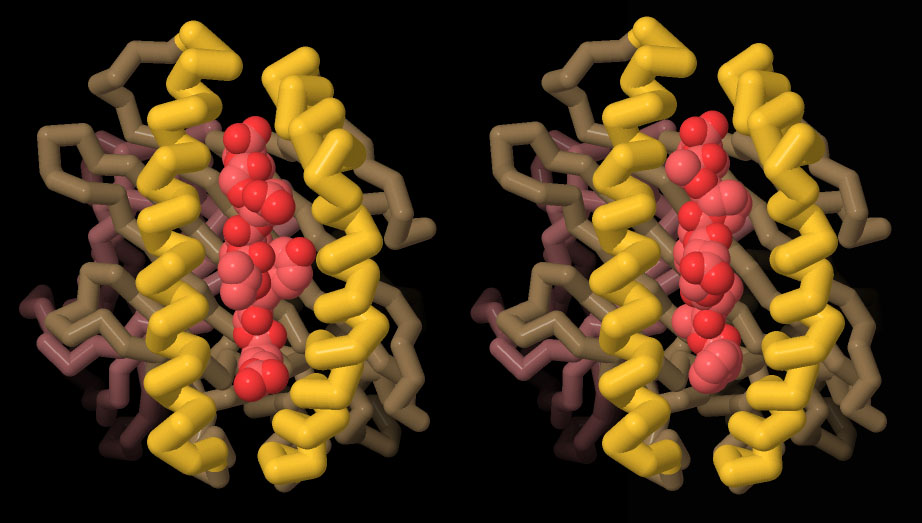
HLA-I Expression and pMHC Tetramer Assembly in E. coli | AtaGenix
HLA-I protein and β2M were expressed and purified in E. coli, followed by refolding to form stable HLA/β2M/peptide complexes (pMHC). The addition of streptavidin (SA) enabled the formation of stable tetramers for downstream applications.
2024-11-05
Protein Expression & Case Studies | AtaGenix Recombinant Protein Solutions
This study demonstrates the optimization of E. coli expression systems for recombinant protein and antibody fragment production. Strategies included host strain selection, periplasmic expression for proper folding, and refolding inclusion bodies into active soluble proteins. Results showcase high-quality protein expression and QC validation.

2024-11-04
Recombinant Antibody Expression | AtaGenix Custom Antibody Services
This case study demonstrates the use of the insect-baculovirus expression system for efficient production of recombinant proteins. The approach achieved high yields with proper folding and post-translational modifications, showcasing its reliability for complex protein expression.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

