AtaGenix Laboratories
AtaGenix Laboratories
2025-08-25
Anti-HE4 Antibody: Cancer Diagnosis Breakthrough | AtaGenix
A 2017 study developed a high-affinity anti-HE4 monoclonal antibody (9C3) using AtaGenix’s advanced mammalian expression and hybridoma platforms. This breakthrough enhances ovarian cancer diagnostics by targeting the HE4 biomarker with high specificity, offering potential for early detection and therapy.
2025-08-25
SARS-CoV-2 Spike Peptides: 2021 Breakthrough in Neutralizing Antibody Research
This article highlights a 2021 Small Methods study that identified immunogenic peptides in the SARS-CoV-2 spike protein and isolated neutralizing monoclonal antibodies from COVID-19 patients. The research pinpointed key RBM epitopes (S431-454, S470-486, S501-515), with S470-486 emerging as an immunodominant target for diagnostics and therapeutics. Using phage display and ScFv libraries, the study validated epitope mapping and antibody functionality. Supported by AtaGenix’s expertise in phage display, protein expression, and assay development, this breakthrough offers a scalable approach for combating emerging viral pathogens, updated as of August 24, 2025.
2025-08-24
Shrimp MANF: Anti-Inflammatory Breakthrough | AtaGenix
This article explores a 2022 peer-reviewed study published in The Journal of Immunology, revealing that Mesencephalic Astrocyte-derived Neurotrophic Factor (MANF) in Litopenaeus vannamei (shrimp) acts as a conserved anti-inflammatory factor. The research demonstrates that extracellular MANF suppresses ERK phosphorylation and downregulates Dorsal (NF-κB) via a receptor-type tyrosine phosphatase (RPTP) in hemocytes, highlighting an ancient immune regulation mechanism with implications for neuroprotection, metabolism, and aging. Cross-species validation in human 293T cells further confirms MANF’s conserved role. Supported by AtaGenix, the study utilized advanced techniques including plasma proteomics, qPCR, and sandwich ELISA with phage display-generated monoclonal antibodies. This breakthrough, detailed as of August 24, 2025, underscores AtaGenix’s role in accelerating innate immunity research through custom antibody discovery, protein expression, and assay development.
2025-10-14
AtaGenix Custom Anti-TauN368 Antibody Enables hTau368 Alzheimer’s Model Research and Uncovers the Pathological Role of Truncated Tau
AtaGenix provides highly specific anti-tauN368 monoclonal antibodies to support Alzheimer’s disease research using the hTau368 transgenic mouse model. These antibodies enable precise detection of truncated tau fragments in Western blot and immunofluorescence experiments, validating tau accumulation, phosphorylation, and associated cognitive deficits in the hippocampus. With exceptional specificity and stability, AtaGenix’s solutions empower researchers to explore tau pathology mechanisms and advance tau-targeted therapeutic development.
2025-10-14
AtaGenix Custom Phospho-specific Antibody Service Facilitates the Discovery of a Novel Mechanism of G Protein-Mediated Growth Regulation in Tomato
The study identified that GGC1 negatively regulates plant growth and photosynthesis by inhibiting CPK28-mediated phosphorylation of PIP1;2 at Thr172. The phospho-specific antibody custom-developed by AtaGenix provided crucial experimental support for elucidating this GGC1–CPK28–PIP1;2 signalling pathway, offering new insights into G protein-mediated regulation of plant growth.

2025-10-13
AtaGenix Custom Proteins Services Enable Breakthrough in Understanding Cholangiocarcinoma Drug Resistance
This study identifies that ROCK2 promotes Pemigatinib resistance in CCA cells via UBA52-mediated DRP1 ubiquitination. AtaGenix provided high-purity custom proteins essential for validating this mechanism.
2025-10-09
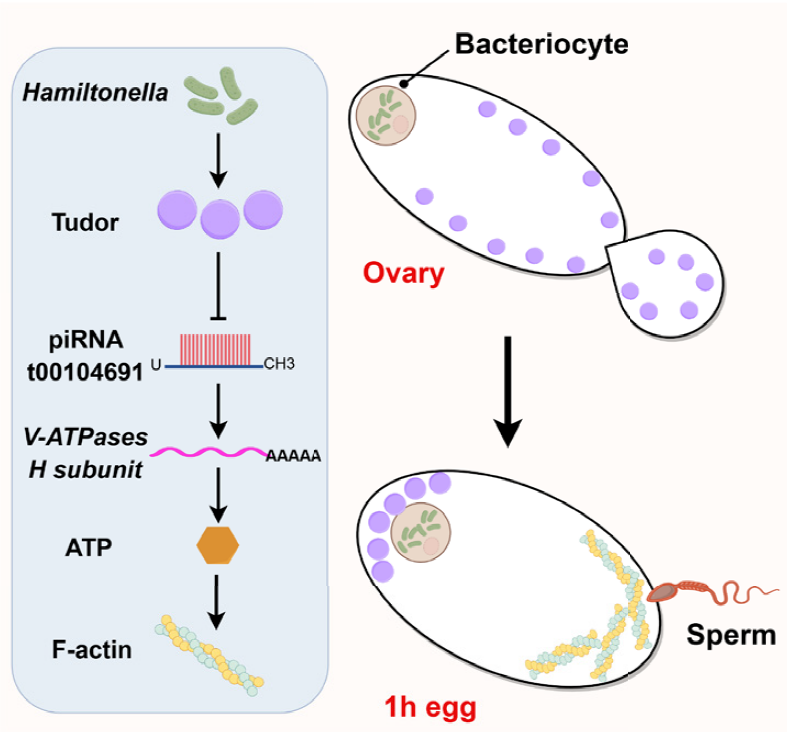
AtaGenix Custom Anti-Tudor Polyclonal Antibody Facilitates the Elucidation of Insect–Microbe Symbiosis Mechanisms
Atagenix offers customised anti-Tudor polyclonal antibody services, addressing challenges of antibody availability and detection specificity in the non-model organism Bemisia tabaci. Through antigen design, antibody production, and multi-level validation, the antibody enables highly specific detection in Western blot and immunofluorescence, providing reliable tools for uncovering how the symbiont Hamiltonella regulates host Tudor protein and reproductive mechanisms.
2025-08-25
AtaGenix Empowers Research on Shrimp Plasma MANF's Anti-Inflammatory Role via Conserved Receptor Tyrosine Phosphatase
This study experimentally identifies shrimp plasma mesencephalic astrocyte-derived neurotrophic factor (MANF) as an LPS-induced protein that inhibits ERK phosphorylation and Dorsal expression in Litopenaeus vannamei hemocytes, elucidating its inflammation regulation mechanism via a conserved receptor tyrosine phosphatase (RPTP). AtaGenix supports the development of high-affinity antibodies, advancing research progress and providing new insights into the evolution of inflammation in invertebrates.
2025-08-20
AtaGenix Customized Phosphorylated Polyclonal Antibodies Unveil the Role of SLKv in Cancer Glycolysis
This pioneering study leverages AtaGenix customized phosphorylated polyclonal antibodies to explore the critical role of the alternatively spliced variant of Ste20-like kinase (SLKv) in enhancing cancer glycolysis and tumor progression. Researchers demonstrated that SLKv boosts enolase 1 (ENO1) activity by binding and phosphorylating its serine 2 residue, significantly increasing phosphoenolpyruvate (PEP) production. This metabolic shift accelerates glycolysis by activating key enzymes such as hexokinase 2 (HK2), phosphofructokinase muscle (PFKM), and phosphoglycerate mutase 1 (PGAM1), with TGFβ-mediated splicing factor KHDRBS1 driving SLK exon skipping. The precision of AtaGenix’s phospho-specific ENO1-S2 antibody enabled accurate quantification of phosphorylation levels, enhancing the study’s reliability. Targeting SLKv with antisense oligonucleotides effectively inhibits glycolysis and tumor growth, marking it as a promising metabolic target for cancer therapy.
2025-08-04
AtaGenix’s Customized ATG8 Antibody Solutions for Studying Amino Acid Metabolism Reprogramming in BmNPV-Infected Silkworms
Bombyx mori nucleopolyhedrovirus (BmNPV) significantly impacts sericulture by reprogramming host amino acid metabolism to support viral replication. AtaGenix provides tailored solutions, including high-specificity ATG8 polyclonal antibodies, to address research challenges in studying BmNPV-induced autophagy and arginine metabolism. These antibodies, validated through immunofluorescence and Western Blot, ensure reliable detection of autophagy markers, aiding researchers in uncovering the virus’s metabolic regulation mechanisms. Supported by advanced gene editing and analytical techniques, our services empower research teams to advance antiviral strategies with agricultural applications.
2025-07-21
AtaGenix Custom rMet Protein Advances Diabetes β-Cell Research
AtaGenix developed high-purity, endotoxin-free recombinant METRNL protein for research teams, uncovering its critical role in preventing β-cell trans-differentiation to α-cells and sustaining islet function, advancing diabetes metabolic stress studies, and offering new therapeutic target insights.
2025-07-14
AtaGenix’s Custom Phospho-USP8 Antibody Advances MDA5 Homeostasis Research, Unveiling a Novel Target for Autoimmune Therapy
A research team from the Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital revealed that phosphorylation of USP8 at Ser718 by AKT regulates the stability of MDA5, a key player in innate antiviral immunity and autoimmune responses. To investigate this mechanism, AtaGenix developed a custom rabbit antibody targeting the phosphorylated Ser718 site on USP8. This high-specificity antibody enabled the team to detect dynamic USP8 activation in cells, confirming its role in MDA5 regulation. The study, published in Advanced Science, provides a new perspective for targeting USP8–MDA5 signaling in autoimmune disease therapy.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

