AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2024-11-05 View volume: 780
Construction of CHO stable cell lines producing recombinant antibodies, with maximum expression levels exceeding 3 g/L.
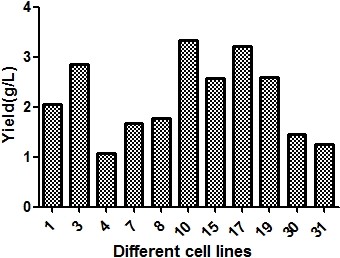
Fig 1. Antibody Monoclonal Yield Analysis. Different clones show yield differences, with top performers exceeding 3 g/L, demonstrating high productivity.
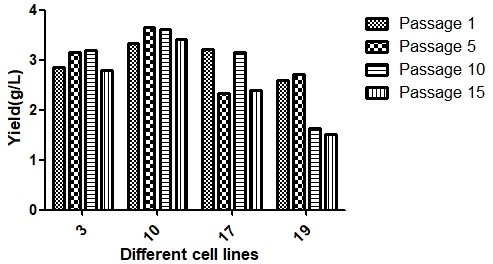
Fig 2. Stability Analysis of Antibody Clones. Expression levels remain consistent across passages, ensuring long-term stability of selected clones.
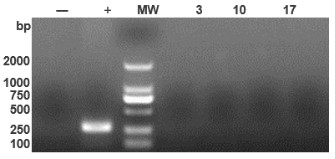
Fig 3. Mycoplasma Testing of Cell Banks (PCR Method). No contamination detected, confirming biosafety and reliability of the CHO cell banks.
MW: DL2000 Marker. +: Positive control (290 bp). -: Negative control.
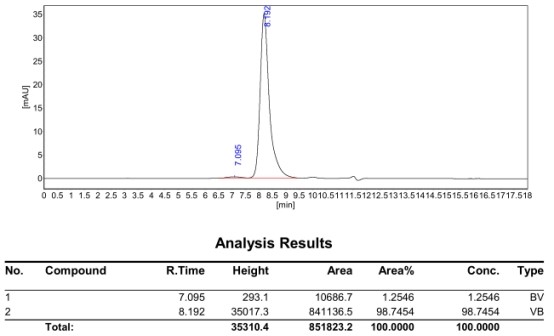
Fig 4. Antibody Purity Analysis of Top1 Cell Line. High purity confirmed by SDS-PAGE, showing minimal impurities and consistent antibody quality.
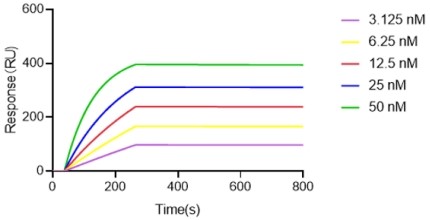
| Sample | ka (1/Ms) | kd (1/s) | KD (M) |
|---|---|---|---|
| 12-10 | 1.29E+05 | 9.52E-06 | 7.36E-11 |
Fig 5. Top1 Cell Line Antibody Affinity Detection. Binding kinetics confirm strong affinity, validating clone selection for therapeutic development.
Expression results of stable cell line producing Varicella-Zoster Virus gE protein.
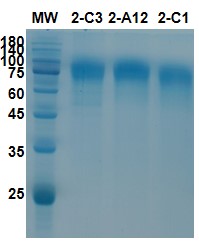
Fig 1. QC SDS-PAGE Analysis of Different Clones. Distinct bands confirm expression of gE protein, supporting downstream vaccine-related studies.
Lane MW: Protein Marker
Need reliable cell line development for antibody or viral protein projects? AtaGenix provides custom solutions from gene design to stable cell bank validation.
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

