AtaGenix Laboratories
AtaGenix Laboratories
Release time: 2026-01-14 View volume: 169
Background
Intrahepatic cholangiocarcinoma (ICC) is an aggressive malignancy with limited long-term survival. U.S. SEER-based statistics report a 10% 5-year relative survival rate across all stages, dropping to 3% in distant-stage disease (2015–2021 cohort), underscoring the urgent need for mechanism-driven therapeutic strategies and patient stratification.
For advanced biliary tract cancers, chemotherapy with gemcitabine plus cisplatin has historically been the standard backbone. Recent phase III trials established chemo-immunotherapy (chemotherapy combined with immune checkpoint blockade) as a clinically meaningful step forward. In TOPAZ-1, adding durvalumab to gemcitabine/cisplatin improved overall survival (hazard ratio 0.80; 95% CI 0.66–0.97) and increased the estimated 24-month overall survival rate (24.9% vs 10.4%). KEYNOTE-966 likewise demonstrated an overall survival benefit with pembrolizumab plus gemcitabine/cisplatin compared with chemotherapy alone. However, resistance remains common, and AKT hyperactivation is prevalent across many tumors, raising a central question: how does AKT-driven metabolism protect tumor cells from immunotherapy-augmented killing?
Study Overview
Published in Cancer Communications (2025), this study interrogates ferroptosis as a resistance node during chemo-immunotherapy in AKT-hyperactivated ICC. The authors performed in vivo metabolic CRISPR screening in a KrasG12D/Tp53-/- ICC mouse model to identify regulators of ferroptosis under a chemo-immunotherapy regimen (gemcitabine + cisplatin + anti–PD-L1 in mice). They pinpointed phosphoenolpyruvate carboxykinase 1 (PCK1) as a key driver and mapped a mechanistic cascade that connects AKT signaling to mevalonate pathway flux, ultimately suppressing lipid peroxidation and ferroptotic cell death.
Importantly, the work extends beyond mechanism discovery: the authors show that pharmacologic interference with the pathway—most notably simvastatin (a mevalonate pathway inhibitor widely used in lipid management)—augmented chemo-immunotherapy efficacy in preclinical models. Clinical correlative analyses further suggest that pAKT–pPCK1 may serve as a biomarker to identify ICC patients more likely to benefit from chemo-immunotherapy.
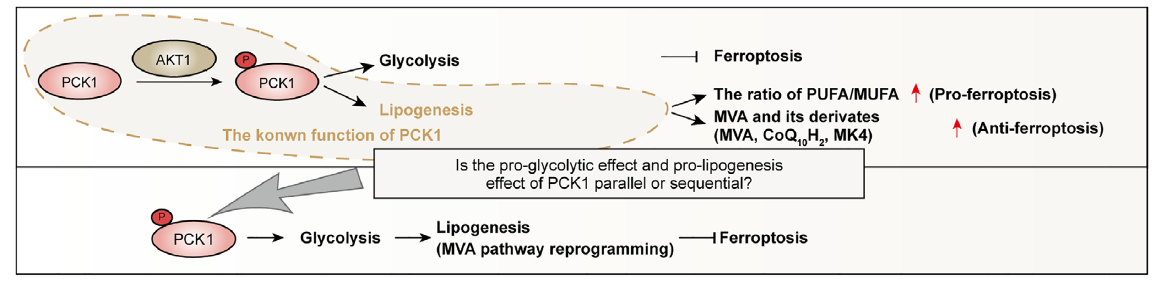
Fig.1 Validation of pPCK1-mediated ferroptosis resistance
Core Mechanism: From AKT Hyperactivation to Ferroptosis Resistance
Ferroptosis is a regulated form of cell death driven by iron-dependent lipid peroxidation. In cancer, ferroptosis intersects with therapeutic response and immune-mediated tumor control, making it a compelling “resistance pressure point.” This study delineates a mechanistic bridge between AKT-driven metabolic rewiring and ferroptosis suppression: AKT phosphorylates PCK1 at Ser90 (pPCK1), and pPCK1 triggers a downstream cascade that increases antioxidant capacity and prevents ferroptotic damage during therapy.
Why Simvastatin Matters: A Repurposing Angle Anchored in Metabolic Vulnerability
The mevalonate pathway supports multiple cellular programs (including lipid biology and antioxidant defense). In this study, interfering with this pathway using simvastatin significantly enhanced chemo-immunotherapy efficacy in preclinical ICC models by disrupting the axis-linked antioxidant shield. Conceptually, this frames AKT-hyperactivated ICC as a tumor subtype with a potentially druggable metabolic dependency: block mevalonate flux → reduce antioxidant buffering → restore lipid peroxidation → re-enable ferroptosis under therapy. Independent research also supports the principle that mevalonate pathway inhibition can sensitize tumor cells to ferroptosis through GPX4-linked mechanisms, reinforcing biological plausibility.
Biomarker Logic: pAKT–pPCK1 as a Response-Stratification Handle
A recurring challenge in chemo-immunotherapy is identifying “who benefits most.” This work provides a mechanistically grounded rationale for stratification: if AKT-driven pPCK1 activation is a prerequisite for mevalonate-mediated ferroptosis resistance, then tumors with elevated pAKT and pPCK1 may represent a subgroup with distinct resistance biology—and therefore a distinct opportunity for combination strategies that target the axis. The paper reports clinical relevance for pPCK1 in AKT-hyperactivated ICC patients receiving chemo-immunotherapy, supporting the idea that pAKT–pPCK1 can function as a predictive biomarker candidate rather than a generic signaling marker.
AtaGenix’s Key Contributions and Results
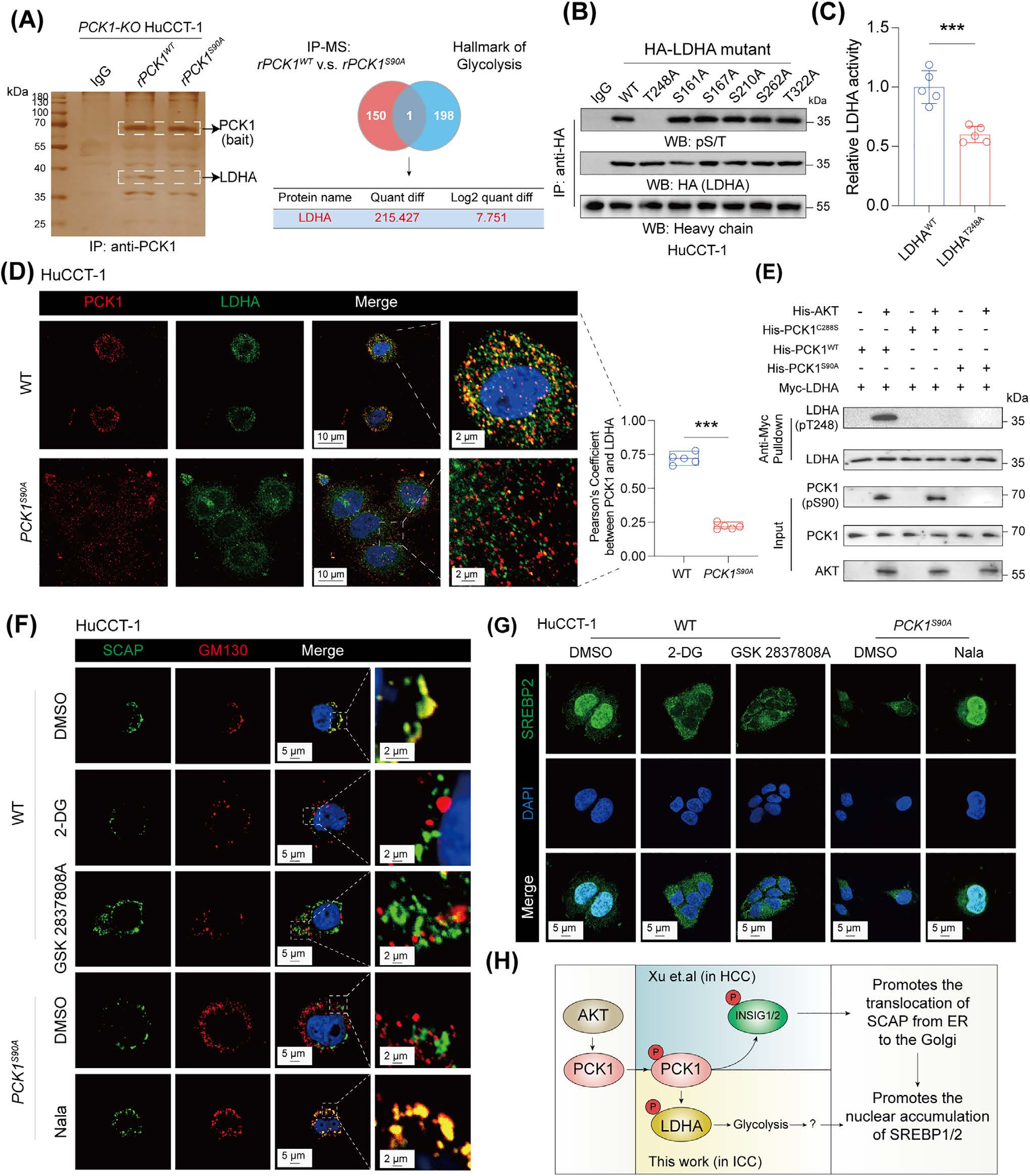
Fig.2 Experimental Results of AtaGenix Antibodies for anti-PCK1 pS90/anti-LDHA pT248 Detection
Recommended Experimental Readouts (Mechanism-to-Data Checklist)
Conclusion
This work establishes the pAKT–pPCK1–pLDHA–SPRINGlac axis as a mechanistic driver of ferroptosis resistance and chemo-immunotherapy resistance in AKT-hyperactivated intrahepatic cholangiocarcinoma. By linking glycolytic activation to mevalonate pathway flux reprogramming and antioxidant shielding, it provides both a drugging concept (axis/mevalonate interference, including statin-based strategies in preclinical models) and a biomarker concept (pAKT–pPCK1) for patient stratification. AtaGenix phospho-specific antibodies (pPCK1 Ser90, pLDHA Thr248) enable high-fidelity detection of the key mechanistic nodes required for replication, biomarker exploration, and translational assay development.
For research use only. This page provides scientific literature interpretation and does not constitute medical advice or treatment recommendations.
References
Contact Us
+86-27-87001869
info@atagenix.com
Building C, R & D Building, No. 666, Shendun 4th Road, Donghu New Technology Development Zone, Wuhan

